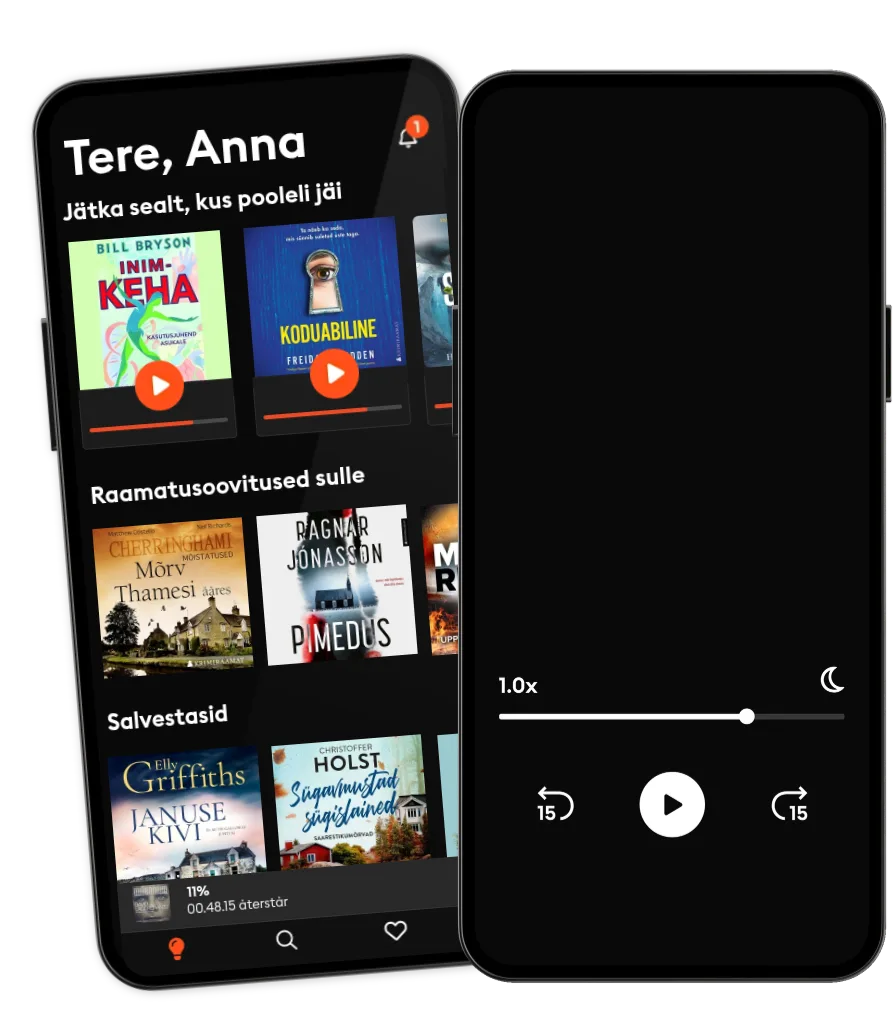
Loe ja kuula
Astu lugude lõputusse maailma
- Proovi tasuta
- Loe ja kuula nii palju, kui soovid
- Suurim valik eestikeelseid raamatuid
- Kokku üle 700 000 raamatu 4 keeles
Analysis of Clinical Trials Using SAS: A Practical Guide, Second Edition
- Autor
- Kirjastaja
- Keel
- inglise
- Vorming
- Kategooria
Teadmiskirjandus
Analysis of Clinical Trials Using SAS®: A Practical Guide, Second Edition bridges the gap between modern statistical methodology and real-world clinical trial applications. Tutorial material and step-by-step instructions illustrated with examples from actual trials serve to define relevant statistical approaches, describe their clinical trial applications, and implement the approaches rapidly and efficiently using the power of SAS. Topics reflect the International Conference on Harmonization (ICH) guidelines for the pharmaceutical industry and address important statistical problems encountered in clinical trials.
Commonly used methods are covered, including dose-escalation and dose-finding methods that are applied in Phase I and Phase II clinical trials, as well as important trial designs and analysis strategies that are employed in Phase II and Phase III clinical trials, such as multiplicity adjustment, data monitoring, and methods for handling incomplete data. This book also features recommendations from clinical trial experts and a discussion of relevant regulatory guidelines.
This new edition includes more examples and case studies, new approaches for addressing statistical problems, and the following new technological updates:
SAS procedures used in group sequential trials (PROC SEQDESIGN and PROC SEQTEST)
SAS procedures used in repeated measures analysis (PROC GLIMMIX and PROC GEE)
macros for implementing a broad range of randomization-based methods in clinical trials, performing complex multiplicity adjustments, and investigating the design and analysis of early phase trials (Phase I dose-escalation trials and Phase II dose-finding trials)
Clinical statisticians, research scientists, and graduate students in biostatistics will greatly benefit from the decades of clinical research experience and the ready-to-use SAS macros compiled in this book.
© 2017 SAS Institute (E-raamat): 9781635261448
Väljaandmise kuupäev
E-raamat: 17. juuli 2017
- Anna O Matthew Blake
5
- Lilledele värsket vett Valérie Perrin
4.9
- Prantsuse kokanduskool Caroline James
4
- Ekraaniaju Anders Hansen
5
- Lapsehoidja Sofie Sarenbrant
4.5
- Loomade farm George Orwell
- Kolgas Max Seeck
4.9
- Tegevjuhi päevik Steven Bartlett
5
- Cinnamon Fallsi saladus R.L. Killmore
4.9
- Kaabaka surm M. C. Beaton
- Must rada Antonio Manzini
- Tüdrukute õhtu – Tove Tönniesi mõrv Lars Olof Lampers
4.3
- Toateenija saladus Nita Prose
4.8
- Neitsi Maarja neli päeva Leelo Tungal
4.3
- Suvi Applemore’is Rachael Lucas
5
- Kallim Freida McFadden
4.8
- Viimne vanne M. W. Craven
4.8
- Lilledele värsket vett Valérie Perrin
4.9
- Anna O Matthew Blake
5
- Suvi Applemore’is Rachael Lucas
5
- Kolgas Max Seeck
4.9
- Koduabiline Freida McFadden
4.9
- Pulmarahvas Alison Espach
4.5
- Õpetaja Freida McFadden
4.7
- Koduabiline jälgib sind Freida McFadden
4.9
- Neitsi Maarja neli päeva Leelo Tungal
4.3
- Koduabilise saladus Freida McFadden
4.7
- Pronksist unelmad Camilla Läckberg
4.5
- Mees, kes teadis ussisõnu Andrus Kivirähk
5
- Apteeker Melchior ja Oleviste mõistatus Indrek Hargla
5
- Kallim Freida McFadden
4.8
- Viimne vanne M. W. Craven
4.8
- Anna O Matthew Blake
5
- Kolgas Max Seeck
4.9
- Suvi Applemore’is Rachael Lucas
5
- Koduabiline Freida McFadden
4.9
- Koduabiline jälgib sind Freida McFadden
4.9
- Pronksist unelmad Camilla Läckberg
4.5
- Pulmarahvas Alison Espach
4.5
- Lapsehoidja Sofie Sarenbrant
4.5
- Koduabilise saladus Freida McFadden
4.7
- Hõbedased tiivad Camilla Läckberg
4.5
- Nukumäng M.W. Craven
4.7
- Tuhat nuga selga Verni Leivak
4.3
- Kadunud sõrmuse mõistatus Ain Kütt
4.3
Vali pakett
Kokku üle 700 000 raamatu 4 keeles
Suur valik eestikeelseid raamatuid
Uusi raamatuid iga nädal
Kids Mode lastesõbralik keskkond
Unlimited
14.99 € /kuus
Tühista igal ajal
Unlimited (aastane)
119.99 € /aasta
Säästa 33%
Eesti
Eesti