Hören und Lesen
Tritt ein in eine Welt voller Geschichten
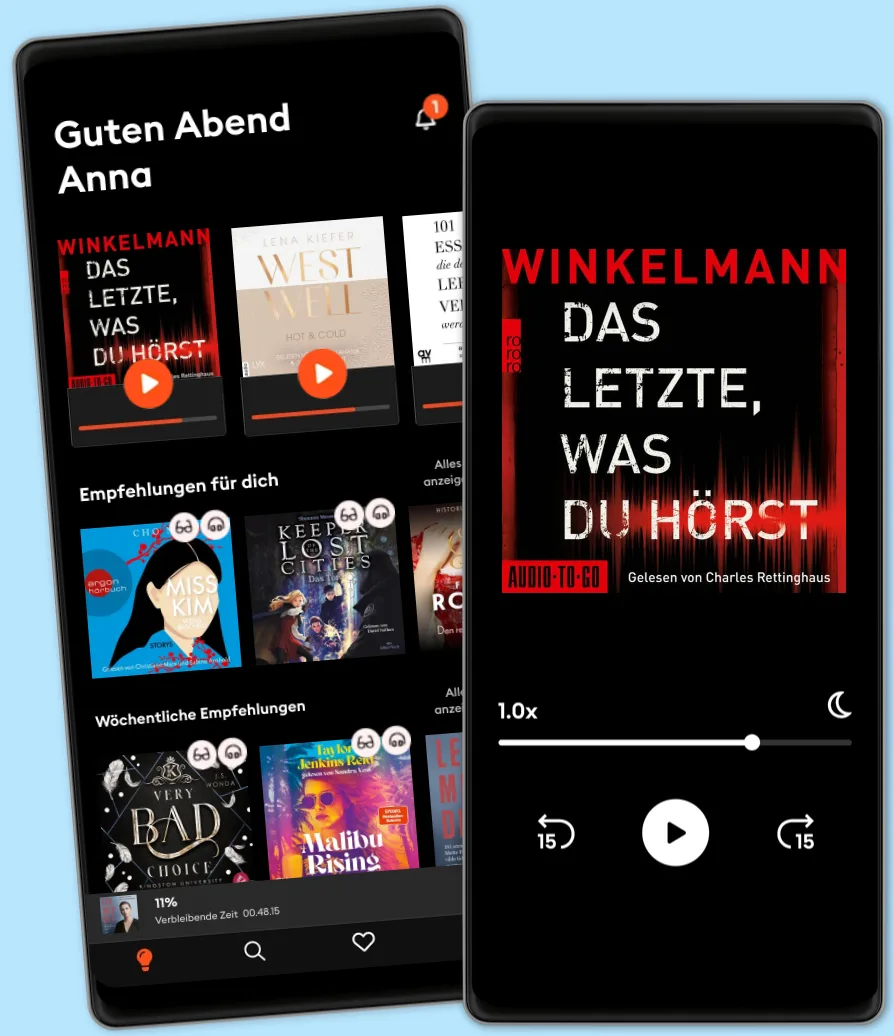
- Mehr als 600.000 Hörbücher und E-Book
- Jederzeit kündbar
- Exklusive Titel und Originals
- komfortabler Kinder-Modus
Implementing ISO/IEC 17025:2017
- Von
- Verlag
- Sprachen
- Englisch
- Format
- Kategorie
Wirtschaft & Karriere
The focus of this book is to demystify the requirements delineated within ISO/IEC 17025:2017, while providing a road map for organizations wishing to receive accreditation for their laboratories.
AS9100, ISO 9001:2015, and ISO 13485:2016 are standards that have been created to support the development and implementation of effective approaches to quality management, and are recognized blueprints for the establishment of a quality management system (QMS) for many diverse industries. Similar to these recognized QMS standards, ISO/IEC 17025:2017 for laboratory accreditation serves a unique purpose. It is not unusual for laboratories to retain dual certification in ISO 9001:2015 and ISO/IEC 17025:2017. However, ISO/IEC 17025:2017 contains requirements specific to the laboratory environment that are not addressed by ISO 9001:2015.
This book highlights those differences between ISO 9001:2015 and ISO/IEC 17025:2017, while providing practical insight and tools needed for laboratories wishing to achieve or sustain accreditation to ISO/IEC 17025:2017. For those currently or formerly accredited to the 2005 version of ISO/IEC 17025, an appendix outlines the changes between the 2005 and 2017 versions of the standard.
© 2019 ASQ Quality Press (E-Book): 9781953079107
Erscheinungsdatum
E-Book: 21. Februar 2019
Tags
Wähle dein Abo-Modell
Über 600.000 Titel
Lade Titel herunter mit dem Offline Modus
Exklusive Titel und Storytel Originals
Sicher für Kinder (Kindermodus)
Einfach jederzeit kündbar
Basic
Für alle, die gelegentlich hören und lesen.
7.90 € /Monat
Jederzeit kündbar
Abo-Upgrade jederzeit möglich
Unlimited
Für alle, die unbegrenzt hören und lesen möchten.
18.90 € /Monat
Jederzeit kündbar
Wechsel zu Basic jederzeit möglich
Anderen gefällt...
- Fourth Wing – Flammengeküsst (Flammengeküsst-Reihe 1) Rebecca Yarros
4.7
- Iron Flame – Flammengeküsst (Flammengeküsst-Reihe 2): Die heißersehnte Fortsetzung des Fantasy-Erfolgs »Fourth Wing« Rebecca Yarros
4.7
- Onyx Storm – Flammengeküsst (Flammengeküsst-Reihe 3): Die heißersehnte Fortsetzung von »Fourth Wing« und »Iron Flame« Rebecca Yarros
4.3
- Rubinrot - Liebe geht durch alle Zeiten - Liebe geht durch alle Zeiten. Die Edelstein-Trilogie, Band 1 (Ungekürzte Lesung) Kerstin Gier
4.6
- The Paradise Problem – Wenn das Herz den perfekten Plan durchkreuzt: Nach »The Unhoneymooners« endlich die neue paradiesisch prickelnde RomCom der SPIEGEL-Bestsellerautorin Christina Lauren
4.4
- The Wrong Bride - The Windsors, Teil 1 (Ungekürzt) Catharina Maura
4.3
- Not Quite Dead Yet (Ungekürzt) Holly Jackson
4.6
- Der dunkle Sommer (Ungekürzte Lesung) Vera Buck
4.7
- The Pumpkin Spice Latte Disaster (Lower Whilby 1): In dieser cosy RomCom treffen Stars Hollow-Vibes auf die Enemies to lover-Trope Kyra Groh
4.4
- "Mama, bitte lern Deutsch" - Unser Eingliederungsversuch in eine geschlossene Gesellschaft (Ungekürzte Autorenlesung) Tahsim Durgun
4.8
- Just for the Summer Abby Jimenez
4.5
- Throne of Glass 1: Die Erwählte Sarah J. Maas
4.6
- Alchemised: Das internationale Phänomen – jetzt auch als Hörbuch SenLinYu
4.3
- Boys of Tommen 5: Taming 7: Die BookTok-Sensation endlich auf Deutsch Chloe Walsh
4.8
- 22 Bahnen: Lieblingsbuch des unabhängigen Buchhandels 2023 Caroline Wahl
4.1
Deutsch
Deutschland